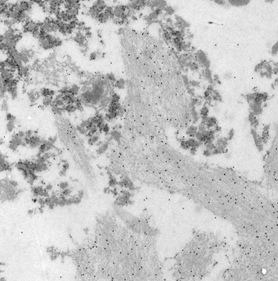
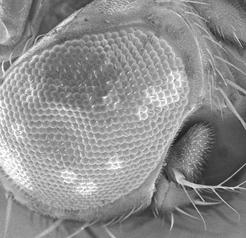
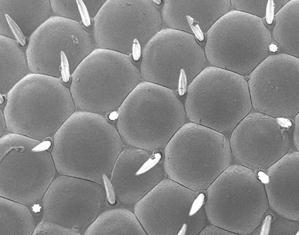
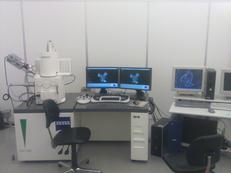
Mineralogy, Petrology and Tectonics research programme at the Department of Earth Sciences combines several related disciplines including classical, environmental and experimental mineralogy, igneous, metamorphic and sedimentary petrology as well as high- and low-temperature geochemistry. We focus on deciphering shallow and deep Earth processes from surface to the Earth’s core. We are operating:
- a JEOL JXA 8530F field emission electron probe microanalyzer equipped with five wavelength dispersive spectrometers and coupled with energy dispersive spectrometer as well as monochromatic cathodoluminescence detector
- WDS and EDS Chemical mapping
- Qualitative WDS scans
- BSE imaging
- SEM imaging
- CL imaging
- Quantitative chemical spot analysis
- Light microscope
- High vacuum carbon coater
- Simple carbon and gold coater
Currently our equipment can be used for a fee and the data obtained are owned by the users. (SEK 4500/day for collaboration projects, 6000/day for external researchers, and 9000/day industrial/commercial users)

Electronmicroprobe laboratory (JXA-8530F JEOL SUPERPROBE)